New RTG1721 Publication: Intracellular transport in 3D
Structural view on the molecular architecture of the co-translational machinery for N-glycosylation
26.03.2018
Roland Beckmann (RTG1721), Katharina Braunger (RTG1721) and coworkers have visualized the complex interplay between protein synthesis, transport and modification.
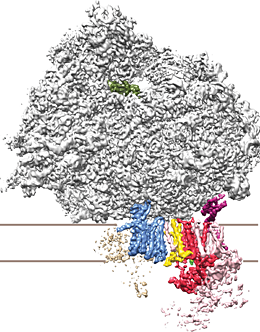
Cells of higher organisms are densely populated by a tubular network of membranes known as the endoplasmic reticulum (ER). ER-resident complexes serve as a binding platform for ribosomes, the cellular machines responsible for protein synthesis. ER-attached ribosomes synthesize proteins destined for various intra- and extracellular locations. To warrant successful delivery, many of these proteins are chemically modified with the molecular equivalent of an address tag upon passage through the ER membrane. LMU researchers led by Professor Roland Beckmann at the Gene Center, in collaboration with colleagues at the Max Planck Institute for Biochemistry, have now determined the three-dimensional structure of the macromolecular complex which couples this labeling-step to ribosomal protein synthesis and protein transport at the ER membrane. Their study provides the basis for a detailed understanding of this vital cellular process and has been published in the journal Science.
Three-dimensional structure of the OST-containing ribosome-translocon complex. Two of the OST subunits (yellow and magenta) link its catalytic subunit (red) to the protein-conducting channel (blue) and the ribosome (gray). Source: K. Braunger, LMU
More information please visit LMU.de/news
Original Publication:
Structural basis for coupling protein transport and N-glycosylation at the mammalian endoplasmic reticulum.
Braunger K, Pfeffer S, Shrimal S, Gilmore R, Berninghausen O, Mandon EC, Becker T, Förster F, Beckmann R.
Science. 2018 Mar 8. pii: eaar7899. doi: 10.1126/science.aar7899

